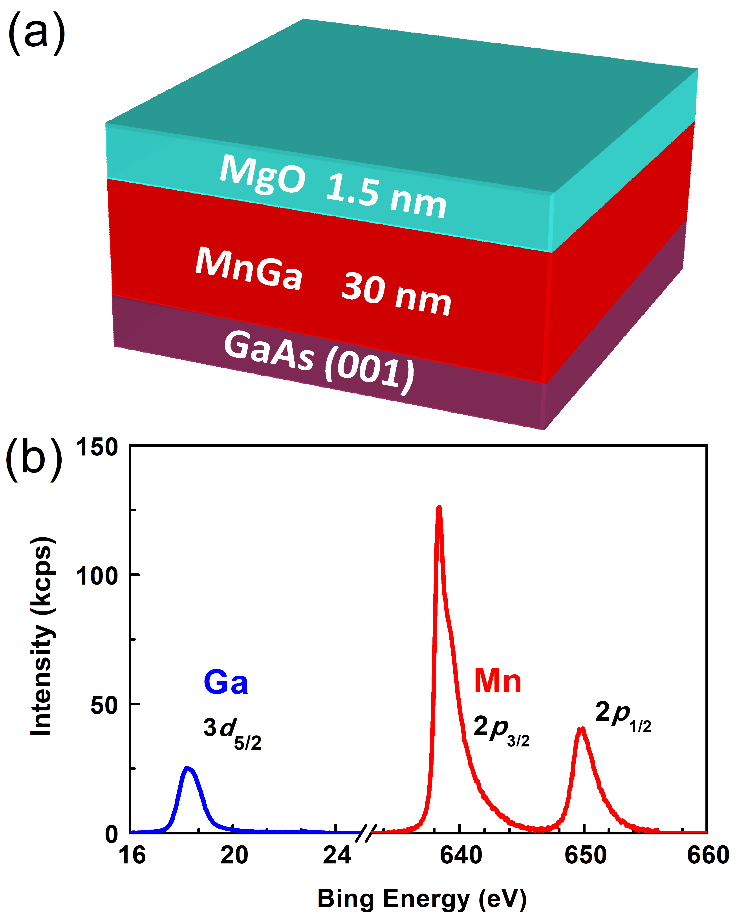
We first exam the chemical stability of MnGa flowing the workflow in Figure 2(a) in acid solutions that are popular in the wet etching process. All the etching tests below were performed at room temperature. A 30 nm MnGa film protected by a 1.5 nm MgO layer was first annealed at 110
oC in vacuum for 20 minutes and then coated with an ultrathin HMDS layer and a 1 µm photoresist of AZ6130 (positive in tone). The patterns were reasonably transferred from the photomask onto the sample surface after prebaking on a hotplate at 100
oC for 10 minutes, ultraviolet exposure for 15 seconds with a Suss MicroTec MA/BA6 Contact Mask Aligner, development in tetramethylammonium Hydroxide (TMAOH) solution (TMAOH:H
2O=4:1) for 30 seconds, rinsing in deionized water for 10 seconds, drying with nitrogen gas flow, post-baking in air furnace at 100
oC for 20 minutes and at 120
oC for 20 minutes, and the removal of the residue resist in oxygen plasma (200 W, 40 seconds). As a reference, we first performed dry etching with argon ion milling (300 V, 0.27 mA/cm
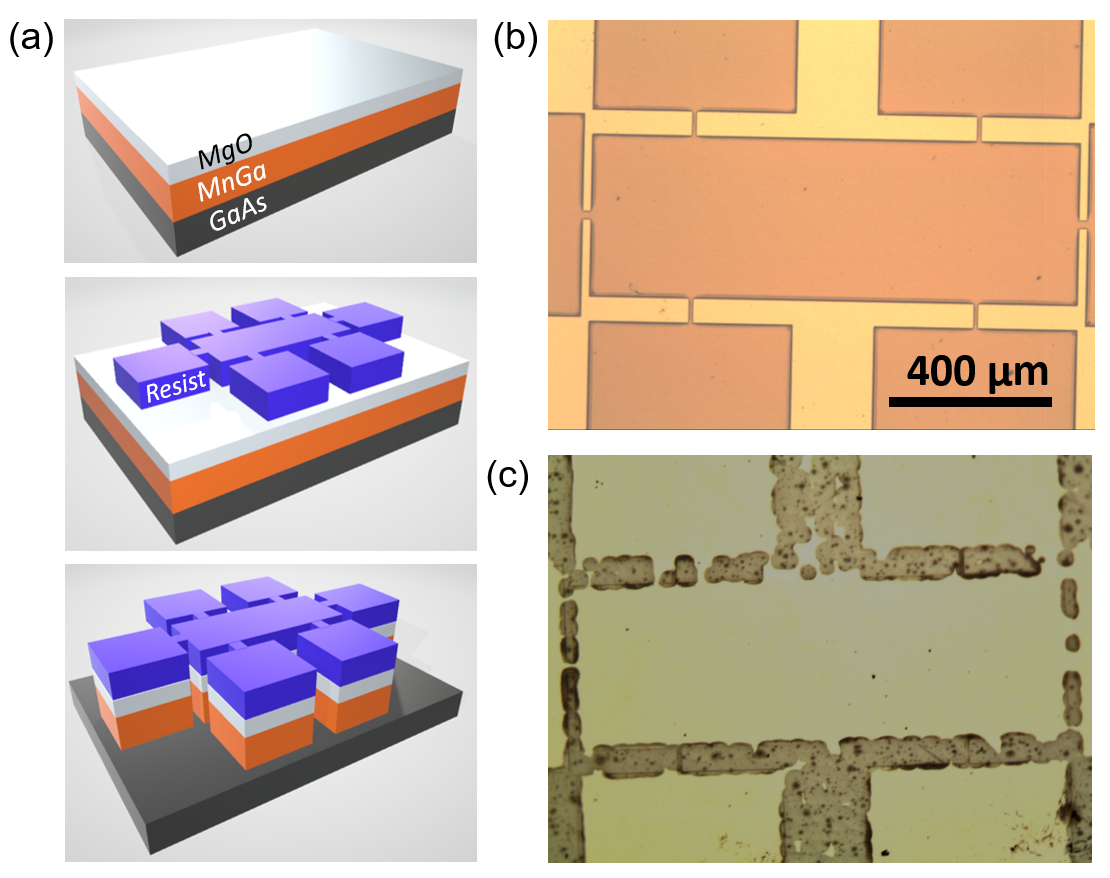
2). As indicated by the optical microscopy imaging in Figure 2(b), the dry etching yields reasonably defined patterns with sharp smooth edges after cleaning off the photoresist with acetone and ethanol. This indicates that the argon-ion milling is a highly selective etching process, with the etching rate much faster in the film normal direction than the in-plane direction. However, the wet etching using acid solutions, i.e., HCl, H
3PO
4 and H
2SO
4 solutions, result in strong sidewall etching, regardless of the combination of different volume ratio and etching time. Figure 2(c) shows the pictures of patterns after wet etching in the solution of HCl:H
2O=1:3, indicating that all the edges are very rough. Some patterns which were supposed to stay was also gone even before the parts which were intended to be etched away still stay on the sample. We also note that some of the connection wires between big pads, which are 10 or 20 µm wide and covered with the resist, were completely etched away even sooner than some of the regions that were not covered with the resist. This observation clearly indicates that the wet etching of MnGa is rather non-uniform with very quick sidewall etching and that the resist seems to be unable to completely protect the MnGa film beneath it, especially in the edge regions. We conclude that acid solutions should be avoided in the fabrication processes of MnGa devices whenever possible. The strong reaction of the MnGa alloys in the acid solutions can be attributed to the different RedOx potentials of Ga
3+/Ga pairs (−530 mV), Mn
2+/Mn pairs (-1185 mV), and H
+/H pairs (0 mV)
[19].
Figure 2.
(a) Schematic depiction of the etching test workflow: the thin film before photolithography (top), resist patterns after photolithography (middle) and patterns after etching (bottom). Optical microscopy images for the MnGa sample surfaces patterned by (b) dry etching and (c) wet etching. 2.3 Chemical Stability in Non-acid Solutions and Oxygen Plasma
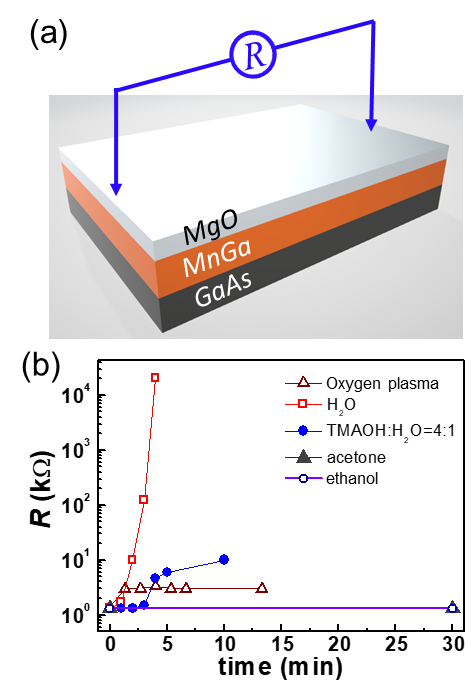
Now we consider the robustness of the MnGa films against the organic solutions, bases, deionized water, and oxygen plasma that are very popular in the standard semiconductor fabrication process. Acetone and ethanol are the most commonly used organic solutions for sample cleaning and lift-off procedures. A typical base for developing the ultraviolet exposed photoresists, such as positive-tone AZ6130 and negative-tone L300, is the TMAOH solution. Deionized water is widely used to rinse samples after photoresist development and to dilute acid or base solutions. Oxygen plasma is usually introduced to etch the resist and to clean the residual resists after photolithography development. Here we use the resistance (R) of the MnGa layer as a measure of the degree of the attack of the MnGa samples in various types of chemical environment (see Figure 3(a) for a schematic of the measurement geometry). The source voltage during the resistance measurements was varied between 1.5 V and 9 V depending on the magnitude of R. In Figure 3(b) we plot the resistance of the 3 mm-wide and 6 mm-long MnGa film pieces with a 1.5 nm MgO capping layer as a function of soaking time in different chemicals. R remains constant in the acetone and the ethanol solutions, indicating that MnGa is not attacked at all by the acetone and ethanol. In striking contrast, in deionized water R increases dramatically with increasing soaking time and reaches 20 MΩ in 5 minutes, revealing a rather violent reaction between MnGa and H2O. Therefore, the use of chemicals containing water should be avoided or reduced during the device fabrication of the MnGa whenever possible. For example, cleaning of the developer off the sample after photolithography development should be done via a quick rinsing rather than a long time soaking in water. In the 75% TMAOH developer, the resistance change of the MnGa sample is negligible in the first 3 minutes, while the extended soaking can increase the resistance by a factor of 10 due to a relatively slow attack. This indicates a short development time, e.g., < 1 min, is not a concern for the MnGa devices. However, a prolonged development process would lead to unwanted etching of the pattern edges and the bare surface. Oxygen plasma with the power of 200 W can oxidize the MnGa surface and passivate the surface in the first 1 min, which indicates that influence of the oxygen plasma can be substantial for an ultrathin MnGa film while negligible for relatively thick films.
Figure 3.
(a) Schematic of the resistance measurement geometry. (b) Evolution of the resistance of a 30 nm MnGa sample in ethanol, acetone, TMAOH developer, deionized water, and oxygen plasma as a function of time.