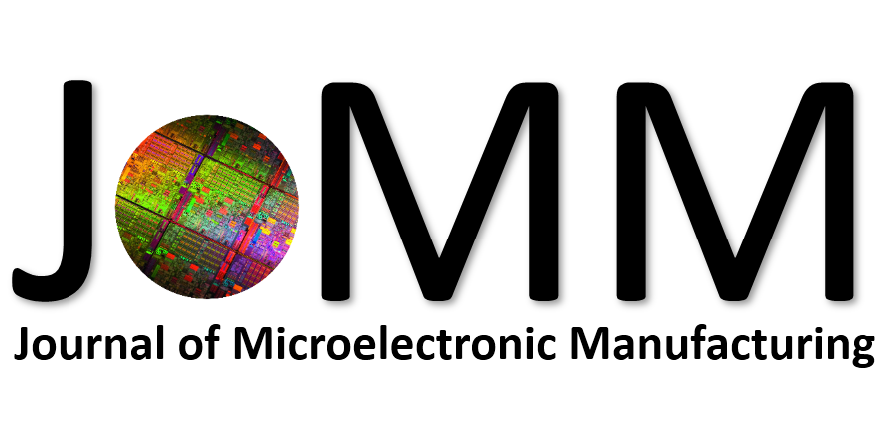
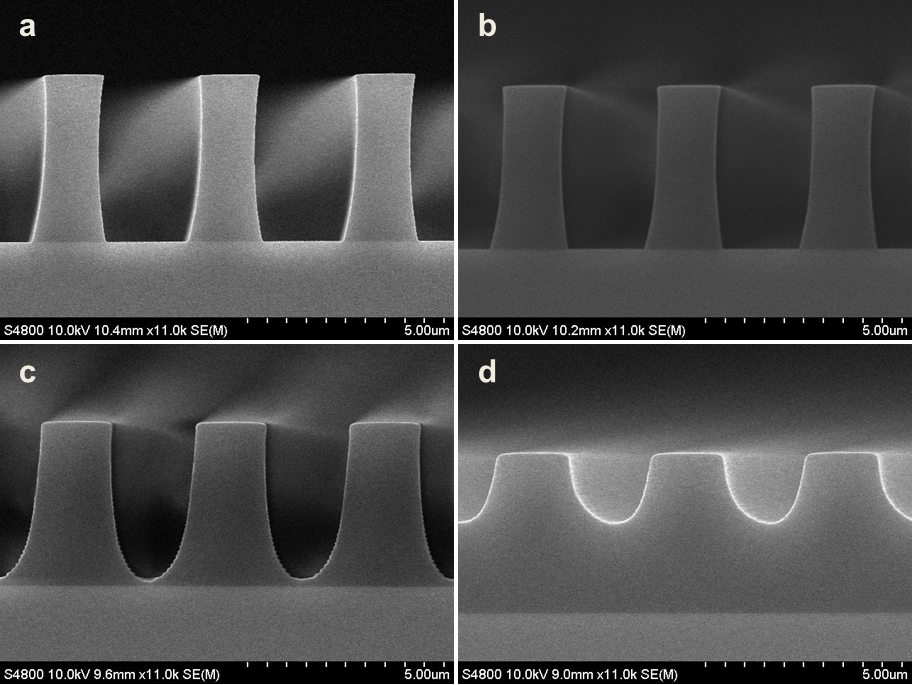
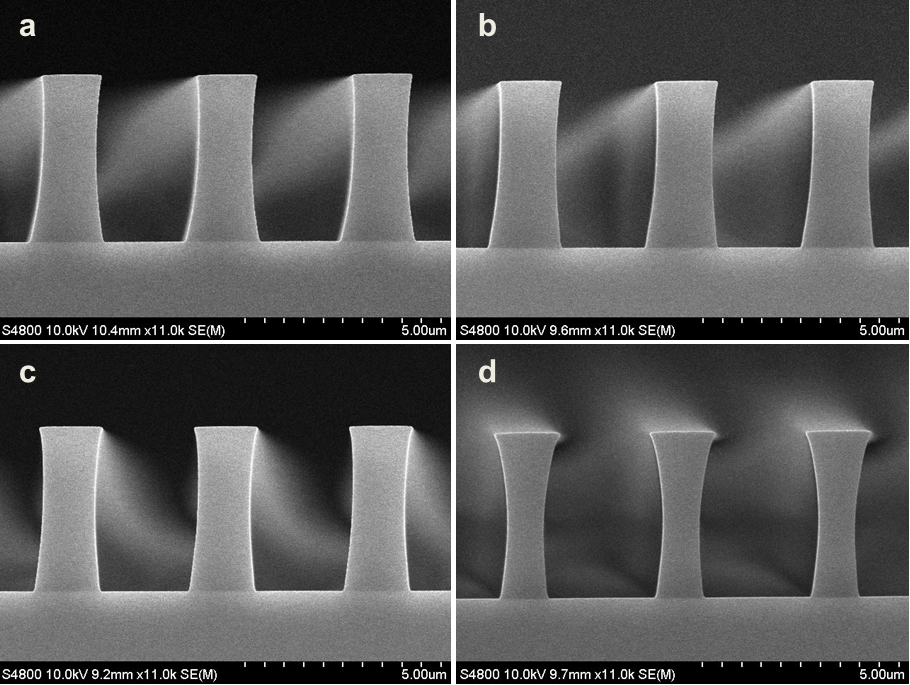
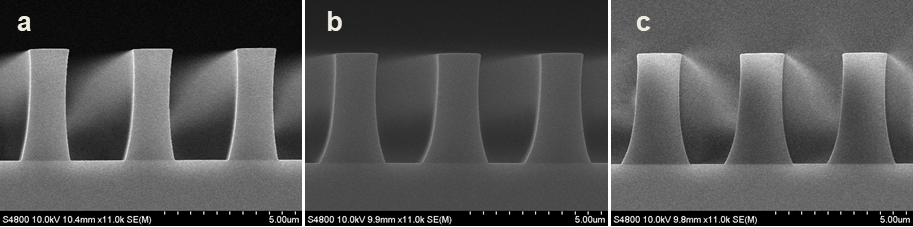
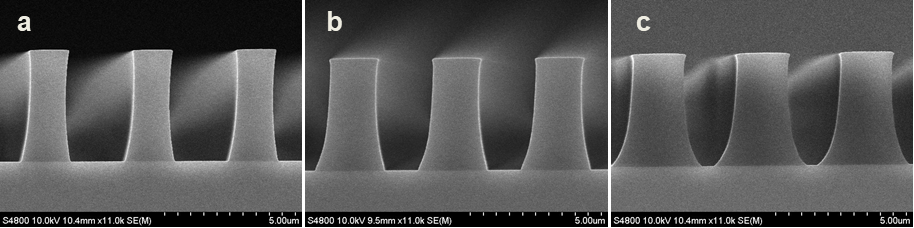
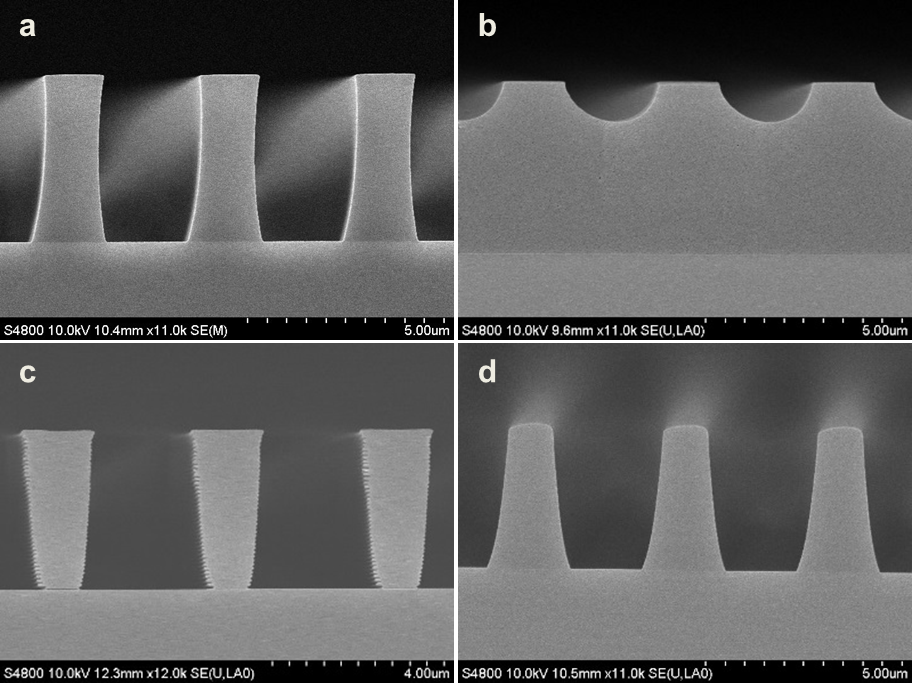
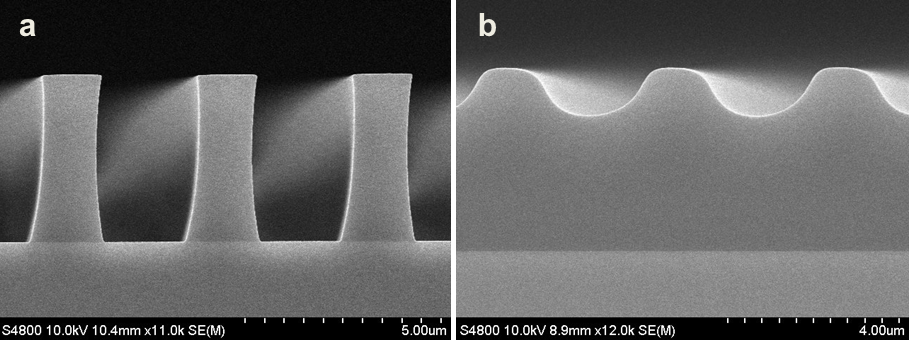
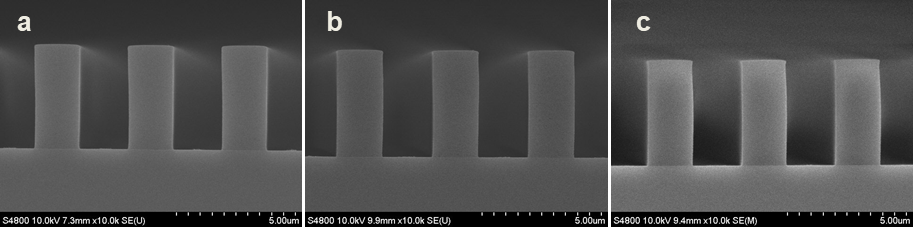
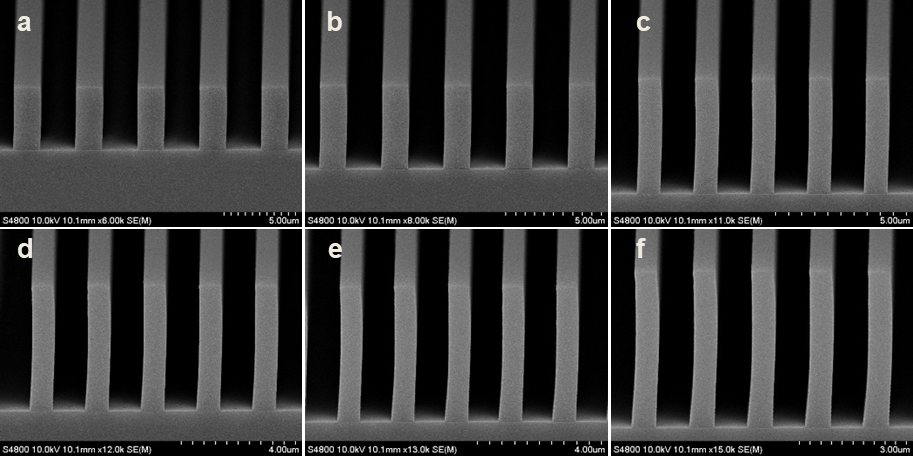
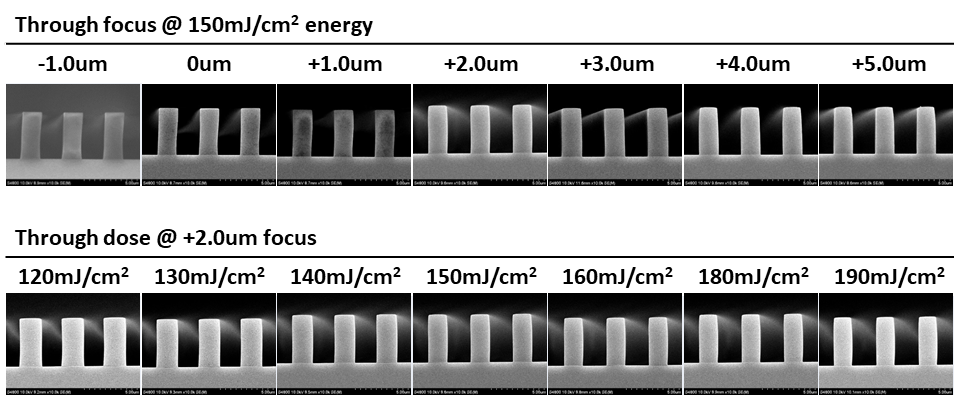
Abstract: An i-Line chemically amplified (ICA) thick film positive resist is reported in this paper. The impact of process conditions on photoresist performance was investigated. Pre-apply bake temperature and post exposure bake temperature affect acid diffusion and deblocking reactions, thus playing an integral role in defining the resist profile. Both pre-apply bake delay and post exposure delay (PED) affect critical dimension (CD) variation, but PED is more sensitive to contact with airborne contaminants. Different polymers and different photo-acid generators (PAG) are also illustrated in this work. By optimizing the structure and concentration of key components, an ICA resist with good environment stability and excellent lithographic performance was demonstrated.
Keywords: Chemical amplification; thick film; i-Line; environment stability; Poly (p-hydroxyl styrene); PAB; PEB